When you pick up a generic pill, you want to know it does the same thing as the brand-name version. That’s where bioequivalence, the scientific proof that two drug formulations produce the same effect in the body. Also known as therapeutic equivalence, it’s the reason your pharmacy can swap out expensive pills for cheaper ones without risking your health. This isn’t guesswork—it’s a strict, measurable standard the FDA uses to approve every generic drug you take.
For a generic drug to be approved, it must release the same amount of active ingredient into your bloodstream at the same rate as the brand-name version. That’s tested in real people through clinical studies, usually comparing blood levels over time. If the results fall within a narrow range—typically 80% to 125% of the original drug—it’s considered bioequivalent. This applies to everything from blood pressure pills to thyroid meds like levothyroxine, where even small differences can cause side effects. The FDA doesn’t just trust the manufacturer’s word; they inspect labs, review data, and sometimes even run their own tests to confirm results.
Bioequivalence doesn’t just protect you—it keeps costs down. Without this standard, generic drugs could be unreliable, and patients might pay more for no real benefit. That’s why you’ll see posts here about how the FDA oversees foreign manufacturing, why stability testing matters for shelf life, and how patent rules affect when generics can enter the market. These aren’t separate topics—they’re all part of the same system that makes sure your $5 generic is just as safe and effective as the $100 brand. Whether you’re managing diabetes with metformin, treating ED with sildenafil, or taking a generic version of Lexapro, bioequivalence is the quiet rule that makes it all work.
Below, you’ll find real-world examples of how this science plays out: from how biosimilars are approved differently than generics, to why switching thyroid meds can cause problems if bioequivalence isn’t properly proven, and how the FDA catches unsafe foreign factories making substandard pills. This isn’t theory—it’s what’s on the shelf, in your medicine cabinet, and in your body.
Generic drugs make up 90% of U.S. prescriptions and save billions yearly. Learn how they're made-from reverse engineering brand-name drugs to FDA-approved manufacturing and bioequivalence testing-without clinical trials.
Population pharmacokinetics uses real-world patient data to prove drug equivalence across diverse populations, replacing traditional bioequivalence studies in many cases. Learn how it works, why regulators accept it, and where it's headed.

Complex generic drugs face major scientific and regulatory hurdles that make approval far harder than for simple generics. Despite high demand and patent expirations, few reach the market due to technical challenges, unclear FDA guidance, and high development costs.
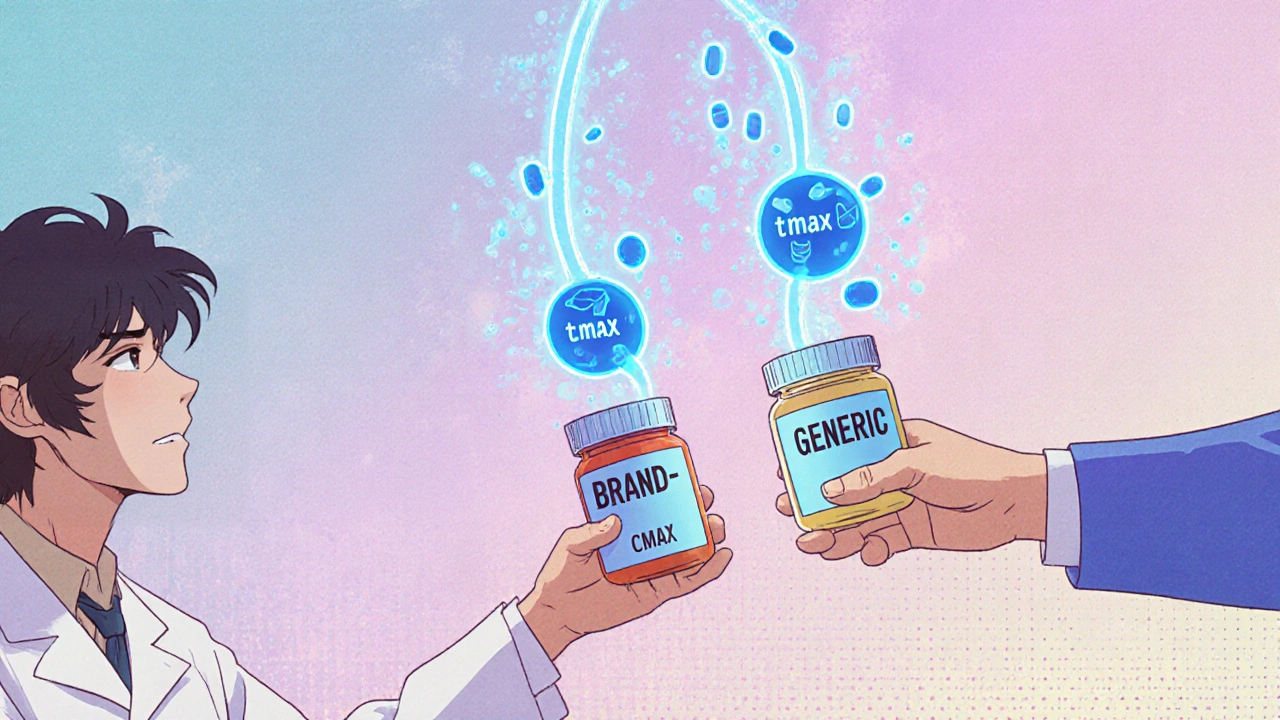
Bioequivalent medications are generic drugs proven to work the same as brand-name versions in your body. Learn how the FDA tests for equivalence, why it matters, and when to ask questions.
