When you pick up a prescription, you might see a generic version instead of the brand-name drug you asked for. That’s not a substitute—it’s often the therapeutic equivalence, the official standard that says a generic drug performs the same way in the body as its brand-name counterpart. Also known as bioequivalence, it’s the reason your pharmacist can swap out expensive pills for cheaper ones without risking your health. The FDA doesn’t just approve generics because they look similar. They require proof—real, measurable proof—that the active ingredient gets into your bloodstream at the same rate and in the same amount. No guesswork. No shortcuts.
This isn’t just about saving money. generic drugs, medications that are chemically identical to brand-name versions but sold under their active ingredient name make treatment possible for millions. Think of levothyroxine for thyroid issues or metformin for diabetes. If you’ve ever switched from one generic to another and felt a difference, that’s not always the drug’s fault. Sometimes it’s about inactive ingredients, timing, or how your body reacts to small variations. But when FDA approval, the rigorous process that ensures drugs meet safety, strength, and quality standards before reaching patients is followed correctly, therapeutic equivalence means you can trust the lower-priced option.
It’s not magic. It’s science. The FDA tests three batches of each generic drug under real and accelerated conditions to prove it won’t break down too fast, lose potency, or behave differently in your body. That’s why you see posts here about stability testing, foreign manufacturing, and patent exclusivity—all part of the same system that keeps generics reliable. You might not know it, but your thyroid medication, your diabetes pill, even your antibiotic, could be part of this invisible quality control network. And when you’re managing chronic conditions like lupus, fatigue, or heart disease, that consistency isn’t optional—it’s life-saving.
Therapeutic equivalence doesn’t mean all generics are the same. Some brands use different fillers or coatings. That’s why switching between generics can sometimes cause issues—even if they’re both approved. That’s why monitoring matters. That’s why your doctor might stick with one brand if you’ve had a good response. But when you’re told a generic is therapeutically equivalent, you can trust it’s held to the same standard as the original. You’re not getting less. You’re getting the same, at a price that makes sense.
Below, you’ll find real-world examples of how this plays out. From how the FDA checks drug factories overseas to why switching thyroid meds needs caution, these posts show you what therapeutic equivalence really looks like in practice—not just in paperwork, but in your body, your wallet, and your daily health routine.
Managing therapeutic equivalence in combination drugs requires more than matching doses. Small formulation differences can change how drugs work together, especially with narrow therapeutic index medications. Learn the risks, real-world cases, and how to stay safe.
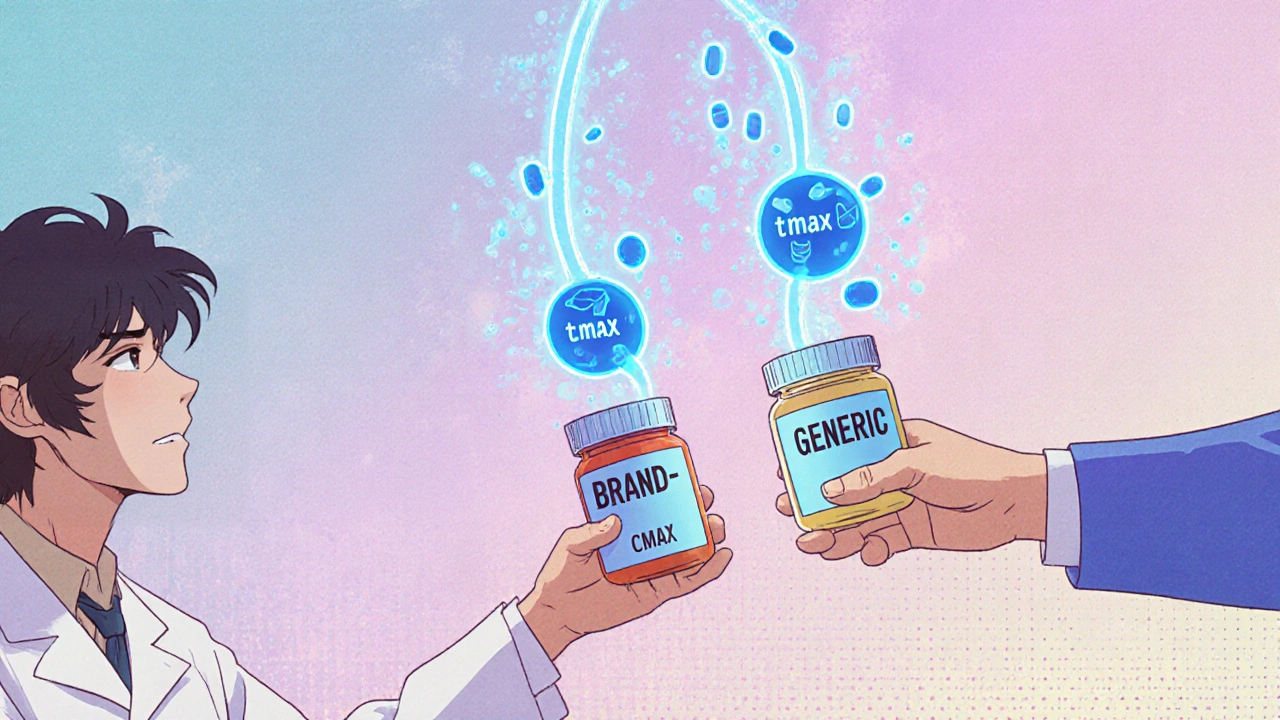
Bioequivalent medications are generic drugs proven to work the same as brand-name versions in your body. Learn how the FDA tests for equivalence, why it matters, and when to ask questions.
