When two or more drugs are combined into a single pill or formulation, getting the dose right isn’t just about matching numbers-it’s about matching effect. Therapeutic equivalence sounds simple: if two products have the same active ingredients, they should work the same. But in combination drugs, that’s where things get messy. A patient switching from a brand-name combo to a generic might get the same milligrams of each drug, but the clinical result? Not always identical.
What Therapeutic Equivalence Really Means
Therapeutic equivalence isn’t just about chemistry. It’s about whether two drug products can be swapped without changing how the patient feels or how safe the treatment is. The FDA’s Orange Book, updated every year, rates over 14,000 drug products with an ‘A’ or ‘B’ code. An ‘A’ rating means the generic is considered interchangeable with the brand. But here’s the catch: this only applies when both products have the exact same active ingredients, strength, dosage form, and route of administration. If one combo pill has a different filler, coating, or release mechanism-even if the active ingredients match-it can still be rated ‘A’… but it might not behave the same in the body.For example, the combination of amlodipine and benazepril is used for high blood pressure. There are at least seven generic versions on the market, all with ‘A’ ratings. But three use croscarmellose sodium as a disintegrant, while four use sodium starch glycolate. These differences don’t show up on the label. Yet, in patients with slow gut motility or those taking multiple meds, even small changes in how fast the pill breaks down can shift blood levels enough to cause dizziness or a spike in blood pressure.
Why Dose Equivalents Don’t Always Add Up
Combination drugs aren’t just the sum of their parts. Sometimes, they work better together than either drug alone-this is called synergy. Take tramadol and acetaminophen. Tramadol alone might reduce pain by 40%. Acetaminophen alone might reduce it by 30%. Together, they don’t just add up to 70%. In some patients, they hit 90%. That’s because tramadol affects opioid receptors, while acetaminophen works on the central nervous system in a different way. Their combined effect isn’t linear.When switching between brands and generics, pharmacists often assume the same total dose equals the same effect. But if the generic uses a different formulation, the release of each component might be delayed or sped up. One study found that switching from brand-name Vytorin (ezetimibe/simvastatin) to a generic version led to a 15% rise in LDL cholesterol in some patients-even though the milligram amounts were identical. Why? The generic’s coating changed how quickly ezetimibe was absorbed. That small delay meant less drug reached the liver at the right time, reducing its cholesterol-lowering effect.

Narrow Therapeutic Index Drugs: The Hidden Danger
Some drugs have a razor-thin line between helping and harming. These are called narrow therapeutic index (NTI) drugs. Warfarin, levothyroxine, phenytoin, and lithium fall into this category. Even a 10% change in blood level can cause a stroke, seizures, or thyroid crisis.When NTI drugs are part of a combination, the risk multiplies. A 2018 study in the Journal of Clinical Endocrinology & Metabolism found that 12% of patients on generic levothyroxine combinations experienced symptoms like fatigue, weight gain, or heart palpitations after switching-even though the FDA deemed them bioequivalent. The problem? The inactive ingredients affected how the thyroid hormone was absorbed. One patient switched from a brand to a generic and went from a TSH level of 2.1 to 7.8 in six weeks. She didn’t know anything was wrong until she collapsed at work.
The FDA requires tighter bioequivalence standards for NTI drugs: 90-111% instead of the usual 80-125%. But that still leaves room for variation. And when you combine two NTI drugs-like a beta-blocker with a diuretic-those tiny differences stack up. A hospital pharmacist in Ohio reported three cases in one year where patients on a combo of metoprolol and hydrochlorothiazide developed low blood pressure after switching generics. The issue wasn’t the dose. It was the timing.
How Substitutions Go Wrong in Real Life
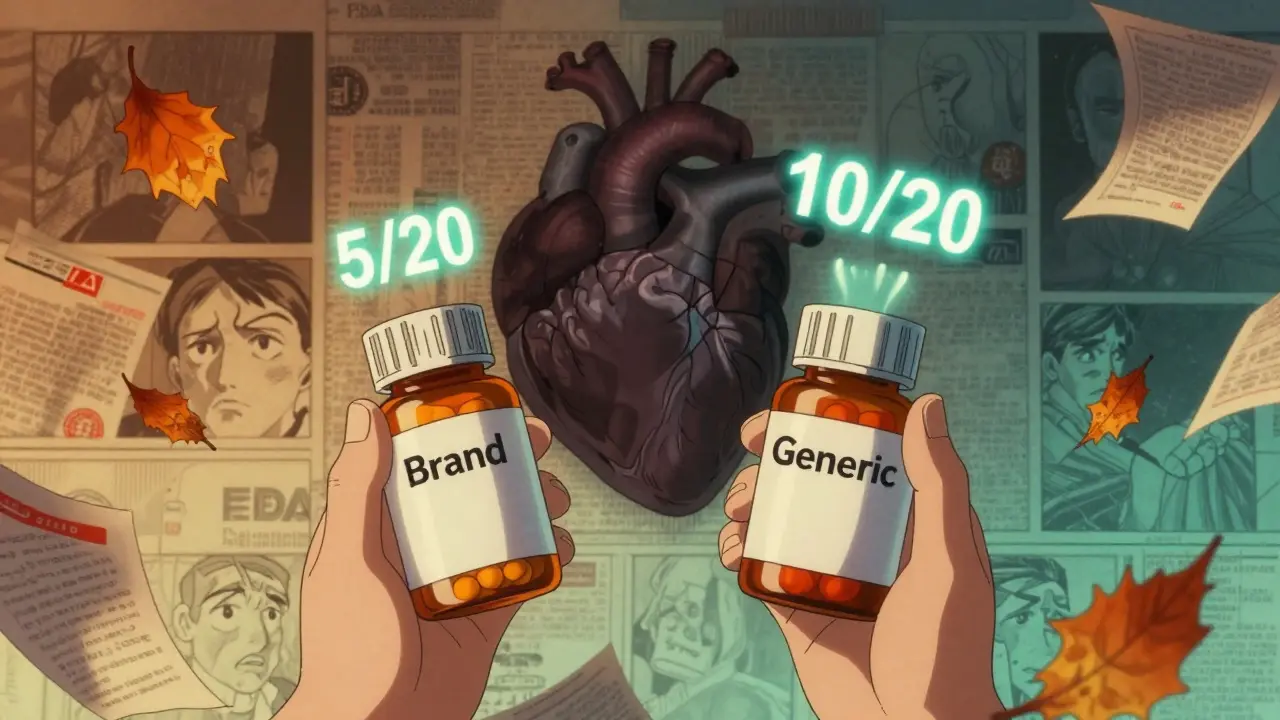
It’s not just about the science. It’s about the system. Pharmacists are under pressure to substitute generics to cut costs. But many don’t have time to check the fine print. A pharmacist on Reddit with 12 years of experience said they made three dosing errors in six months just switching between different strengths of amlodipine/benazepril combos. Why? The pills looked the same. The names were similar. The only difference was a tiny ‘5/20’ vs. ‘10/20’ printed on the bottle.On Allnurses.com, a nurse practitioner shared a case where a patient on a combo of sertraline and olanzapine for depression switched to a generic. Within days, the patient became agitated and had trouble sleeping. The doses were technically equivalent. But the generic used a different salt form of sertraline, which changed how fast it entered the bloodstream. The patient’s brain couldn’t adjust. It took three weeks and a switch back to the brand to stabilize.
Meanwhile, the FDA’s adverse event database recorded 247 incidents in 2022 tied to dose conversion errors in combination products. Nearly 40% involved heart or psychiatric drugs. That’s not a glitch. It’s a systemic blind spot.

What Works: How to Manage This Safely
There are ways to reduce the risk. Hospitals that use standardized conversion tables for NTI combos have cut errors by over 60%. Barcode scanning before dispensing helps catch mismatches. Some systems now require a 72-hour follow-up after any switch involving a combination drug with an NTI component.At the University of California Health System, a 40-hour training program for pharmacists and nurses reduced substitution errors by two-thirds. The training didn’t just teach the rules-it showed real cases. One slide showed a patient who went from a brand to a generic combo of valsartan and hydrochlorothiazide. The generic used a different polymer coating. The patient’s blood pressure dropped too low because the drug was absorbed too fast. She ended up in the ER. That’s the kind of story that sticks.
Another solution? Don’t assume all ‘A’ rated products are equal. If a patient is stable on a specific brand or generic, keep them on it. Switching for cost savings isn’t worth the risk if the patient is doing well. The savings from switching a single pill might be $5 a month. The cost of a hospital visit? Thousands.
The Future: Personalized Equivalence
The FDA is working on new tools to predict when a generic combo might fail. They’re using machine learning to analyze formulation differences and flag high-risk substitutions. Early tests show 89% accuracy. That’s promising. But it’s not enough.The real shift is coming from personalized medicine. By 2030, experts predict that 30% of therapeutic equivalence decisions will include genetic data. Someone who metabolizes drugs slowly due to a CYP2D6 gene variant might need a lower dose of one component in a combo, even if the standard dose is ‘equivalent’ for most people. Right now, that’s science fiction. But it’s the direction we’re heading.
For now, the message is clear: therapeutic equivalence is a useful tool-but it’s not a guarantee. When it comes to combination drugs, especially those with NTI components, the same dose doesn’t always mean the same outcome. Patients deserve more than a barcode scan and a cost-saving checkbox. They deserve thoughtful, individualized care.
Can I safely switch between generic and brand-name combination drugs?
It depends. For most combination drugs without narrow therapeutic index (NTI) components, switching is generally safe if both products have an ‘A’ rating in the FDA’s Orange Book. But for drugs like levothyroxine, warfarin, or phenytoin-especially in combos-even small formulation differences can cause problems. If a patient is stable, it’s often safer to stay on the same version. Always consult the prescriber before switching.
Why do two ‘A’ rated generics of the same combo work differently?
Because therapeutic equivalence only requires identical active ingredients and strength. Inactive ingredients-like fillers, coatings, and disintegrants-can vary between manufacturers. These affect how fast the drug dissolves and is absorbed. In some patients, especially those with digestive issues or on multiple meds, that small difference can change blood levels enough to cause side effects or reduced effectiveness.
Are combination drugs more likely to have therapeutic equivalence issues?
Yes. Combination drugs are more complex because each component can be affected differently by formulation changes. One drug might be absorbed faster, while the other lags. Synergistic effects can be disrupted. NTI drugs in combos are especially risky. Studies show 72% of psychiatric combos have lower therapeutic equivalence reliability than statins, largely due to complex pharmacokinetics.
What should I do if I notice side effects after switching a combination drug?
Don’t ignore it. Track your symptoms-note when they started, what they feel like, and whether they match any changes in your meds. Contact your prescriber immediately. Bring the new pill bottle and the old one if you still have it. Many side effects from generic switches are reversible if caught early. Never stop a combo drug abruptly, especially if it includes a blood pressure or psychiatric medication.
How can I find out if my combo drug is rated ‘A’?
Check the FDA’s Orange Book online. Search by the brand name or active ingredients. Look for the ‘TE Code’-if it starts with ‘A’, it’s rated therapeutically equivalent. But remember: ‘A’ doesn’t mean identical. It means the FDA considers it interchangeable based on current standards. If you’re on an NTI drug or have had issues before, ask your pharmacist if the generic you’re getting is the same one you’ve used before.